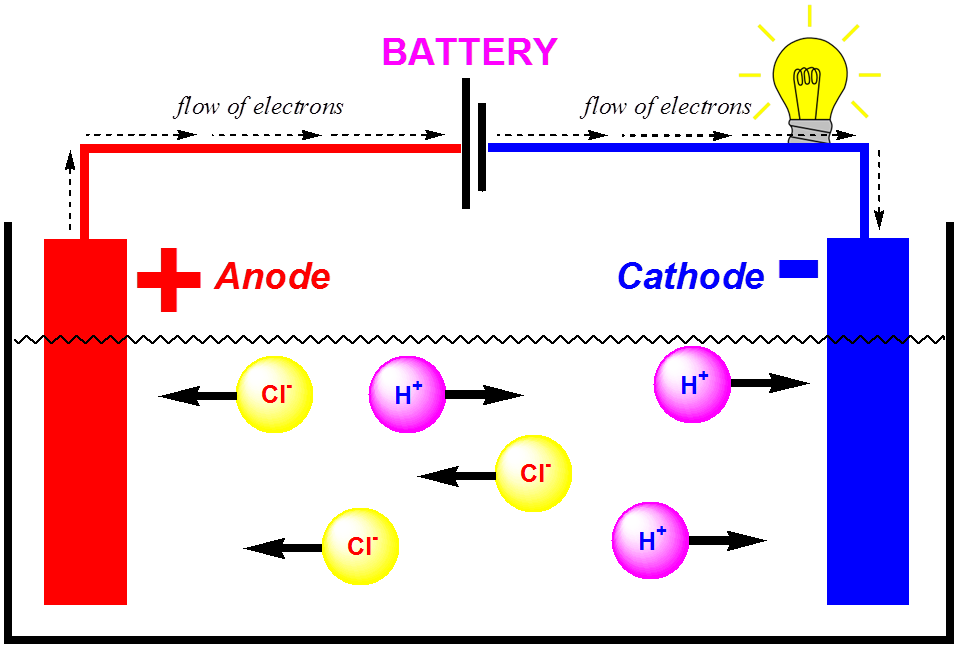
How Electrolyte Solutions Conduct Electricity . molar conductivity is a key property of an electrolyte solution that measures how well it conducts electricity. an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). in some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react. describe some of the major ways in which the conduction of electricity through a solution differs from metallic. these solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. in some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules. in order to conduct electricity, a solution needs to have ions (charged particles) to carry electrical charge from one electrode. The larger the concentration of ions, the.
from chemistryfromscratch.org
The larger the concentration of ions, the. describe some of the major ways in which the conduction of electricity through a solution differs from metallic. an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). molar conductivity is a key property of an electrolyte solution that measures how well it conducts electricity. in order to conduct electricity, a solution needs to have ions (charged particles) to carry electrical charge from one electrode. in some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules. in some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react. these solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively.
V2.8
How Electrolyte Solutions Conduct Electricity The larger the concentration of ions, the. in some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules. The larger the concentration of ions, the. in order to conduct electricity, a solution needs to have ions (charged particles) to carry electrical charge from one electrode. molar conductivity is a key property of an electrolyte solution that measures how well it conducts electricity. describe some of the major ways in which the conduction of electricity through a solution differs from metallic. these solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. in some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react. an electrolyte solution conducts electricity because of the movement of ions in the solution (see above).
From www.nagwa.com
Question Video Identifying Which of Three Substances Is a Strong How Electrolyte Solutions Conduct Electricity describe some of the major ways in which the conduction of electricity through a solution differs from metallic. The larger the concentration of ions, the. in some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react. in some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules.. How Electrolyte Solutions Conduct Electricity.
From www.britannica.com
Electric current in a solution of electrolytes studied Britannica How Electrolyte Solutions Conduct Electricity an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). describe some of the major ways in which the conduction of electricity through a solution differs from metallic. these solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. in. How Electrolyte Solutions Conduct Electricity.
From www.youtube.com
Which of the following best explains why electrolytes solutions conduct How Electrolyte Solutions Conduct Electricity in order to conduct electricity, a solution needs to have ions (charged particles) to carry electrical charge from one electrode. these solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. molar conductivity is a key property of an electrolyte solution that measures how well it conducts. How Electrolyte Solutions Conduct Electricity.
From www.teachoo.com
Do liquids conduct electricity? Class 8 Science Notes Teachoo How Electrolyte Solutions Conduct Electricity The larger the concentration of ions, the. in some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules. an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). molar conductivity is a key property of an electrolyte solution that measures how well it conducts electricity.. How Electrolyte Solutions Conduct Electricity.
From www.slidegeeks.com
Electrolyte Solutions Conduct Electricity In Powerpoint And Google How Electrolyte Solutions Conduct Electricity in order to conduct electricity, a solution needs to have ions (charged particles) to carry electrical charge from one electrode. in some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react. describe some of the major ways in which the conduction of electricity through a solution differs from metallic. The larger the concentration. How Electrolyte Solutions Conduct Electricity.
From www.galleon.ph
Jieoto Water Electrolysis Apparatus Electrolyte Solutions Conduct How Electrolyte Solutions Conduct Electricity in some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react. in order to conduct electricity, a solution needs to have ions (charged particles) to carry electrical charge from one electrode. describe some of the major ways in which the conduction of electricity through a solution differs from metallic. The larger the concentration. How Electrolyte Solutions Conduct Electricity.
From slideplayer.com
ELECTROLYTES Substances that when dissolved in water will conduct How Electrolyte Solutions Conduct Electricity these solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. in some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules. in order to conduct electricity, a solution needs to have ions (charged particles) to carry electrical charge from one. How Electrolyte Solutions Conduct Electricity.
From www.vrogue.co
Electrolyte Nonelectrolyte Solutions Infographic Diag vrogue.co How Electrolyte Solutions Conduct Electricity in some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules. molar conductivity is a key property of an electrolyte solution that measures how well it conducts electricity. these solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. in. How Electrolyte Solutions Conduct Electricity.
From www.numerade.com
SOLVEDWhen asked what causes electrolyte solutions to conduct How Electrolyte Solutions Conduct Electricity The larger the concentration of ions, the. in some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react. describe some of the major ways in which the conduction of electricity through a solution differs from metallic. in some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules.. How Electrolyte Solutions Conduct Electricity.
From www.desertcart.lk
Buy Water Electrolysis Apparatus Electrolyte Solutions Conduct How Electrolyte Solutions Conduct Electricity in some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules. these solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. molar conductivity is a key property of an electrolyte solution that measures how well it conducts electricity. describe. How Electrolyte Solutions Conduct Electricity.
From chm2046l.weebly.com
Electric Solutions CHM 2046L How Electrolyte Solutions Conduct Electricity these solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). describe some of the major ways in which the conduction of electricity through a solution differs from metallic. molar. How Electrolyte Solutions Conduct Electricity.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors How Electrolyte Solutions Conduct Electricity in order to conduct electricity, a solution needs to have ions (charged particles) to carry electrical charge from one electrode. in some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules. molar conductivity is a key property of an electrolyte solution that measures how well it conducts electricity. these solutions. How Electrolyte Solutions Conduct Electricity.
From slideplayer.com
ELECTROLYTES Substances that when dissolved in water will conduct How Electrolyte Solutions Conduct Electricity The larger the concentration of ions, the. in order to conduct electricity, a solution needs to have ions (charged particles) to carry electrical charge from one electrode. molar conductivity is a key property of an electrolyte solution that measures how well it conducts electricity. in some cases, solutions prepared from covalent compounds conduct electricity because the solute. How Electrolyte Solutions Conduct Electricity.
From psu.pb.unizin.org
17.7 Electrolysis Chemistry 112 Chapters 1217 of OpenStax General How Electrolyte Solutions Conduct Electricity an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The larger the concentration of ions, the. in some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules. these solutions conduct electricity due to the mobility of the positive and negative ions, which are called. How Electrolyte Solutions Conduct Electricity.
From materialmcgheepearter.z21.web.core.windows.net
Can Salt Water Conduct Electricity How Electrolyte Solutions Conduct Electricity molar conductivity is a key property of an electrolyte solution that measures how well it conducts electricity. these solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. in some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react. in some cases,. How Electrolyte Solutions Conduct Electricity.
From energyeducation.ca
Electrolyte Energy Education How Electrolyte Solutions Conduct Electricity in some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react. these solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. in some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules. an electrolyte solution. How Electrolyte Solutions Conduct Electricity.
From www.tessshebaylo.com
Water Undergoes Electrolysis Equation Tessshebaylo How Electrolyte Solutions Conduct Electricity in some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react. molar conductivity is a key property of an electrolyte solution that measures how well it conducts electricity. describe some of the major ways in which the conduction of electricity through a solution differs from metallic. an electrolyte solution conducts electricity because. How Electrolyte Solutions Conduct Electricity.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes How Electrolyte Solutions Conduct Electricity an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). describe some of the major ways in which the conduction of electricity through a solution differs from metallic. these solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. The larger. How Electrolyte Solutions Conduct Electricity.